Epidural steroid injections
Commentary by Connie Deline, MD
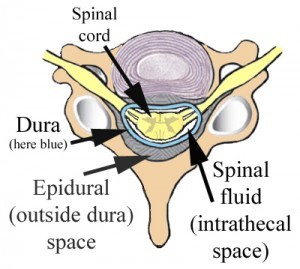
Epidural corticosteroid injections have been used increasingly for pain related to spinal pathology including spinal stenosis and radiculopathy. The epidural space is the space inside the spinal canal but outside of the dura (see image).
However, many are unaware of the fact that the use of corticosteroids in the epidural space is off-label, meaning that this particular use is not FDA approved. Risks and benefits should be weighed, as in any procedure.
Risks from epidural steroid injections
Increased attention to the risks of these procedures came about after several hundred patients developed fungal infections (meningitis, epidural abscess, osteomyelitis) in 2012 as a result of contaminated vials of steroid from a single compounding pharmacy. Tragically, some of these infections resulted in death.
Overall, serious complications associated with epidural steroid injections appear to be rare but include:
- infections (bacterial, fungal, viral) (meningitis, abscesses, osteomyelitis)
- neurologic complications such as nerve root injury, spinal cord injury, stroke
- epidural or subdural hematoma
- pneumocephalus
- avascular necrosis of femoral head (destruction of ball of hip joint)
Inadvertent dural puncture resulting in post-dural puncture headache is often considered to be a less serious complication despite the fact that a small subset of these will require open surgical repair when epidural blood patches are not effective. The severe positional headache is often very disabling. These iatrogenic (meaning “the result of medical procedure”) spinal CSF leaks are very likely under-reported.
Research: Meta-analysis
The meta-analysis below reviewed the results of 38 randomized, placebo-controlled trials that sought to evaluate the effectiveness of epidural steroid injections for spinal stenosis or radiculopathy. No short-term or long-term benefit was found in spinal stenosis. In radiculopathy, a small short-term benefit in pain and need for surgery was found, but there was no long-term benefit. They found that serious harm was reported infrequently, but acknowledge under-reporting.
Like any procedure, the risks versus benefits must be carefully weighed by the attending physician and the patient when considering epidural steroid injections. Serious risks associated with epidural steroid injections, even when rare, must be weighed against the limited potential for benefit.
Study
AUTHORS: Chou R, Hashimoto R, Friedly J, Fu R, Bougatsos C, Dana T, Sullivan SD, Jarvik J.
CITATION: Ann Intern Med. 2015 Sep 1;163(5):373-81. doi: 10.7326/M15-0934.
BACKGROUND: Use of epidural corticosteroid injections is increasing.
PURPOSE: To review evidence on the benefits and harms of epidural corticosteroid injections in adults with radicular low back pain or spinal stenosis of any duration.
DATA SOURCES: Ovid MEDLINE (through May 2015), Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, prior systematic reviews, and reference lists.
STUDY SELECTION: Randomized trials of epidural corticosteroid injections versus placebo interventions, or that compared epidural injection techniques, corticosteroids, or doses.
DATA EXTRACTION: Dual extraction and quality assessment of individual studies, which were used to determine the overall strength of evidence (SOE).
DATA SYNTHESIS: 30 placebo-controlled trials evaluated epidural corticosteroid injections for radiculopathy, and 8 trials were done for spinal stenosis. For radiculopathy, epidural corticosteroids were associated with greater immediate-term reduction in pain (weighted mean difference on a scale of 0 to 100, -7.55 [95% CI, -11.4 to -3.74]; SOE, moderate), function (standardized mean difference after exclusion of an outlier trial, -0.33 [CI, -0.56 to -0.09]; SOE, low), and short-term surgery risk (relative risk, 0.62 [CI, 0.41 to 0.92]; SOE, low). Effects were below predefined minimum clinically important difference thresholds, and there were no longer-term benefits. Limited evidence showed no clear effects of technical factors, patient characteristics, or comparator interventions on estimates. There were no clear effects of epidural corticosteroid injections for spinal stenosis (SOE, low to moderate). Serious harms were rare, but harms reporting was suboptimal (SOE, low).
LIMITATIONS: The review was restricted to English-language studies. Some meta-analyses were based on small numbers of trials (particularly for spinal stenosis), and most trials had methodological shortcomings.
CONCLUSION: Epidural corticosteroid injections for radiculopathy were associated with immediate reductions in pain and function. However, benefits were small and not sustained, and there was no effect on long-term surgery risk. Limited evidence suggested no effectiveness for spinal stenosis.
PMID: 26302454
DOI: 10.7326/M15-0934